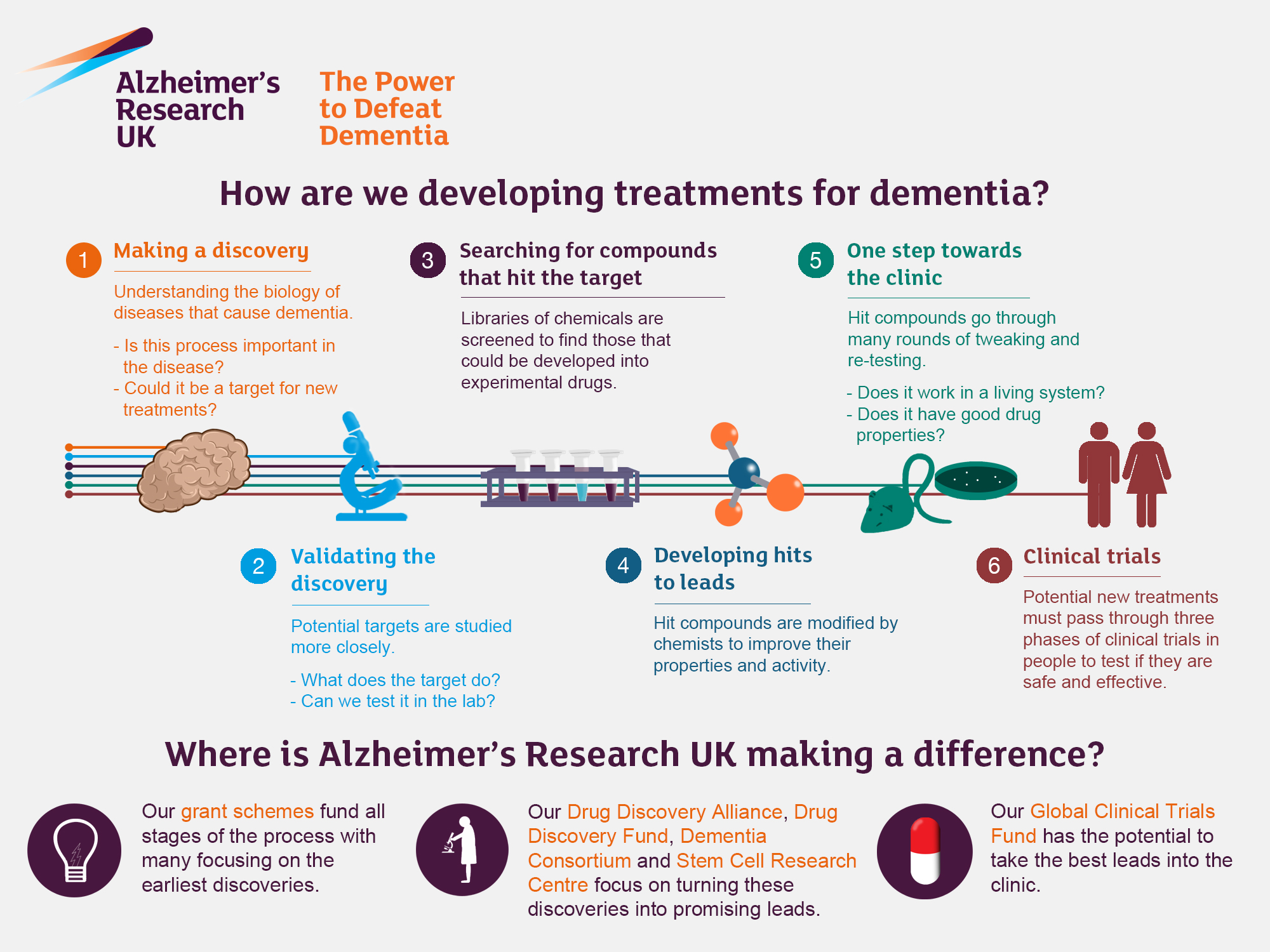
Alzheimer’s research has opened new frontiers in our understanding of this devastating condition that currently affects millions. Led by pioneering neuroscientist Beth Stevens, investigators are delving into the crucial role of microglial cells, the brain’s own immune system. These cells not only defend against degenerative diseases but also participate in synaptic pruning, the process through which the brain fine-tunes neural connections. However, when this process goes awry, it may contribute to the development of Alzheimer’s and other neurodegenerative diseases, raising the stakes for innovative therapeutic interventions. With ongoing NIH funding supporting these vital studies, hopes are high for new biomarker discoveries and potential treatments that could profoundly improve the lives of those impacted by this illness.
Exploring the realm of cognitive decline, research focused on Alzheimer’s disease reveals a complexity within the brain that is increasingly understood through the lens of immune function. The investigation into microglial cells, essential components of the brain’s immune landscape, highlights their role in maintaining synaptic integrity and combating neurodegeneration. Studies spearheaded by experts like Beth Stevens unfold the importance of these cells in the context of illnesses like Alzheimer’s and Huntington’s disease. As scientists explore these intricate relationships, the link between the brain’s immune response and the progression of neurodegenerative disorders becomes ever clearer. Thanks to substantial backing from federal agencies, this groundbreaking research paves the way for advancements that may someday transform care for millions suffering from cognitive impairments.
Understanding the Role of Microglial Cells in Brain Health
Microglial cells, often dubbed the brain’s immune system, play a pivotal role in maintaining neurological health and homeostasis. These specialized cells are responsible for constant surveillance of the brain’s microenvironment, detecting signs of injury or disease, and responding accordingly. In doing so, microglial cells actively engage in synaptic pruning, a process that optimizes neural connections by removing excess or dysfunctional synapses. This activity is crucial not only for normal brain development but also for the maintenance of cognitive functions throughout life.
However, the functionality of microglia can sometimes go awry, contributing to the pathogenesis of neurodegenerative diseases such as Alzheimer’s. Aberrant pruning activity of these immune cells may lead to synapse loss, which is a hallmark of dementia and other related disorders. Understanding the dual role of microglial cells—both beneficial and harmful—opens avenues for new therapeutic strategies aimed at modulating their activity in neurodegenerative conditions.
Beth Stevens: Pioneering Research in Alzheimer’s Disease
Beth Stevens, a leading figure in Alzheimer’s research, has significantly influenced our understanding of how microglial cells affect neurodegeneration. Her work at the Stevens Lab has illuminated the connections between neuroinflammation and synaptic pruning, emphasizing the importance of the brain’s immune response in diseases like Alzheimer’s. With generous support from NIH funding, her research team has identified biomarkers that could revolutionize early detection and treatment options for Alzheimer’s, benefiting millions affected by this pervasive disease.
Stevens’ groundbreaking findings highlight that the mechanisms underlying synaptic pruning by microglial cells can lead to harmful outcomes when misregulated. This pivotal research spans over two decades and echoes the importance of basic science research as the foundation for practical applications in medicine. By elucidating the links between microglial behavior and neurodegenerative diseases, Stevens is paving the way for innovative treatment strategies that could transform the landscape of Alzheimer’s care.
The Importance of NIH Funding in Alzheimer’s Research
The backbone of impactful Alzheimer’s research often stems from NIH funding, which enables scientists like Beth Stevens to pursue critical questions without the immediate pressure of producing commercially viable results. This support has allowed researchers to delve into fundamental aspects of brain health and disease mechanisms, leading to significant advancements in our understanding of neurodegenerative conditions. Without this investment, many of the discoveries that fuel our current biomedical understanding may never have been made.
NIH funding not only facilitates research into Alzheimer’s disease but also empowers scientists to explore related topics, such as the role of microglial cells, neuroinflammation, and synaptic dynamics. Such interconnected areas of study are vital for a holistic understanding of brain health and disease. By fostering curiosity-driven research, the NIH plays an instrumental role in bridging the gaps between basic science, translational research, and clinical practice, ultimately aiming for breakthroughs that improve patient outcomes.
Neurodegenerative Diseases: The Intersection of Microglia and Synaptic Dynamics
Neurodegenerative diseases, including Alzheimer’s, pose a significant challenge to public health. Recent findings have pointed to the critical roles that microglial cells play in these diseases, particularly in processes like synaptic pruning. When microglia become dysregulated, they may contribute to the loss of synapses vital for cognitive function, leading to the symptoms observed in patients with neurodegenerative conditions. This underscores the complex interplay between the brain’s immune system and neuronal health, making it a focal point for future research.
Understanding the mechanisms of synaptic pruning mediated by microglial cells allows researchers to investigate potential therapeutic targets that could alleviate the effects of neurodegeneration. For instance, if microglial activity can be modulated to enhance their protective functions while preventing harmful pruning, this could significantly alter the trajectory of diseases like Alzheimer’s. Innovative research in this area may uncover new pathways for intervention, potentially altering the course of neural degeneration and improving quality of life for patients.
Research Gains Traction: Connecting Synaptic Pruning and Neurodegeneration
As the connection between synaptic pruning and neurodegenerative diseases becomes clearer, the implications for research funding and focus areas are significant. Understanding how microglial cells contribute to both neuroprotection and neurodegeneration opens the door to novel intervention strategies. Researchers are now examining how therapeutic modulation of microglial activity might enhance cognitive functions while protecting against synapse loss in diseases like Alzheimer’s.
The exploration of this dynamic relationship is fostering a new generation of research that not only links basic science to clinical applications but also advocates for sustained investment in neurobiological research. By elucidating how microglia interact with neurons during both health and disease, investigators are laying the groundwork for targeted therapies that can offer hope to those affected by neurodegenerative disorders while informing future funding priorities.
Innovative Biomarkers in Alzheimer’s Research
The quest for effective biomarkers in Alzheimer’s research is advancing at a rapid pace, largely due to the efforts of scientists like Beth Stevens. Biomarkers that can reliably indicate the presence or progression of Alzheimer’s disease are critical for early diagnosis and monitoring treatment efficacy. Stevens’ groundbreaking research on microglial cells and their role in synaptic pruning has led to the discovery of potential biomarkers that could revolutionize how we diagnose and manage Alzheimer’s and other neurodegenerative diseases.
By linking inflammatory responses of microglial cells to neurodegeneration, researchers can develop assays that detect these biomarkers in biological samples. The identification of such markers not only aids in understanding the disease process but also enhances the ability to evaluate new therapies in clinical trials. The integration of these findings into clinical practice holds promise for improving outcomes, underscoring the importance of ongoing investment in Alzheimer’s research and development.
The Future of Alzheimer’s Treatment: New Therapeutic Approaches
As research into microglial cells continues to evolve, the potential for discovering new therapeutic approaches for Alzheimer’s disease becomes increasingly promising. Therapies aimed at modulating microglial functions may offer novel avenues for treatment, either by enhancing the protective roles of these immune cells or by inhibiting their detrimental activities. By focusing on the balance of microglial functions, researchers are seeking to develop strategies that not only prevent synapse loss but also promote neuroprotection.
Moreover, innovative research designs that incorporate insights from basic and clinical science can lead to more effective therapies. By targeting specific pathways involved in inflammation and synaptic pruning, new drugs could help mitigate the impacts of neurodegenerative diseases. The integration of multidisciplinary research efforts, supported by continued federal funding, is crucial for advancing the development of these promising interventions that could change the landscape of Alzheimer’s treatment.
The Intersection of Basic Science and Practical Applications in Neuroscience
The relationship between basic science and practical applications in neuroscience is pivotal for understanding complex diseases like Alzheimer’s. Researchers like Beth Stevens illustrate how fundamental investigations into microglial cells lead to real-world implications, creating a bridge between laboratory findings and clinical insights. These basic research projects not only contribute to our fundamental knowledge but also inspire the development of innovative diagnostic tools and therapeutic strategies.
The synergy between curiosity-driven research and translational science is essential for addressing the pressing challenges posed by neurodegenerative diseases. By continuing to support groundbreaking research through agencies like the NIH, we ensure that discoveries in cellular and molecular biology can be translated into effective treatments that directly impact patient care. This integration of knowledge from different scientific domains propels us closer to fulfilling the promise of personalized medicine in the fight against Alzheimer’s.
The Role of Synaptic Plasticity in Cognitive Health
Synaptic plasticity, the ability of synapses to strengthen or weaken over time, is crucial for learning, memory, and overall cognitive function. The role of microglial cells in modulating synaptic plasticity adds a layer of complexity to our understanding of brain health. Research indicates that microglia not only participate in synaptic pruning but also support the formation of new synaptic connections, highlighting their dual role in promoting cognitive resilience. Enhancing synaptic plasticity through targeted interventions may provide a promising strategy for maintaining cognitive health as we age.
Understanding the intricate relationships between synaptic plasticity, microglial function, and neurocognitive disorders is a dynamic area of research. Scientists are investigating how alterations in synaptic plasticity can lead to cognitive decline in conditions like Alzheimer’s disease. By elucidating these mechanisms, researchers aim to devise therapies that bolster synaptic health and cognitive function, ultimately contributing to a better quality of life for individuals facing neurodegenerative challenges.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are essential components of the brain’s immune system, playing a crucial role in Alzheimer’s research. They monitor brain health by clearing out damaged cells and regulating synaptic pruning, which is vital for healthy neural communication. Abnormal function of these cells can lead to neurodegenerative diseases such as Alzheimer’s.
How does understanding synaptic pruning contribute to Alzheimer’s research?
Understanding synaptic pruning is vital in Alzheimer’s research as it reveals how microglial cells manage neural connections. Aberrant synaptic pruning can exacerbate neurodegenerative diseases, highlighting potential therapeutic targets for treating Alzheimer’s and improving cognitive function.
Why is Beth Stevens significant in Alzheimer’s research?
Beth Stevens is significant in Alzheimer’s research due to her pioneering work on microglial cells and their function in synaptic pruning. Her research elucidates the mechanisms of brain immune responses, which could lead to innovative treatments for neurodegenerative diseases, including Alzheimer’s.
How does NIH funding impact Alzheimer’s research?
NIH funding is crucial for Alzheimer’s research as it supports foundational studies that may seem distant from immediate clinical applications. This funding enables scientists, like Beth Stevens, to explore key areas, such as microglial function and synaptic pruning, paving the way for future breakthroughs in treating Alzheimer’s and other neurodegenerative diseases.
What are the implications of aberrant pruning in neurodegenerative diseases?
Aberrant pruning by microglial cells has significant implications in neurodegenerative diseases, including Alzheimer’s. By disrupting the normal clearance of synapses, this process can lead to cognitive decline and neurodegeneration, making it a focal point for research aiming to develop new therapeutics and biomarkers.
What discoveries have been made about microglial cells in relation to Alzheimer’s?
Recent discoveries about microglial cells have revealed their critical roles beyond just immune response; they are involved in synaptic pruning and neuroinflammation. These insights are central to Alzheimer’s research, showing how altered microglial behavior can contribute to synaptic loss and cognitive impairment, forming potential targets for intervention.
How do neurodegenerative diseases relate to brain immune systems?
Neurodegenerative diseases, including Alzheimer’s, are closely related to the brain’s immune system, particularly through the function of microglial cells. These cells respond to injury and disease by managing synaptic pruning and inflammation. Dysregulation of this immune response can exacerbate conditions like Alzheimer’s, highlighting the need for more research in this area.
What kind of breakthroughs have emerged from Beth Stevens’ research on Alzheimer’s?
Breakthroughs from Beth Stevens’ research on Alzheimer’s involve identifying the role of microglial cells in synaptic pruning and their implications in neurodegenerative diseases. These findings have the potential to lead to novel biomarkers and treatment strategies that could significantly impact how Alzheimer’s disease is managed and understood.
| Key Point | Details |
|---|---|
| Role of Microglia | Microglia act as the brain’s immune system, clearing damaged cells and pruning synapses. |
| Impact on Alzheimer’s Disease | Aberrant pruning by microglia can contribute to Alzheimer’s and other neurodegenerative diseases. |
| Research Foundation | Beth Stevens’s research, funded largely by NIH, is vital for understanding and treating Alzheimer’s. |
| Progress and Potential | Stevens’s work paves the way for new biomarkers and treatments that can impact millions living with Alzheimer’s. |
Summary
Alzheimer’s research is progressing significantly, as highlighted by the innovative work of Beth Stevens and her team. Their exploration into microglial cells has reshaped our understanding of how the brain’s immune system interacts with neurodegenerative diseases. As they uncover the complexities of microglial pruning and its implications for Alzheimer’s, researchers are developing promising biomarkers and therapies. This research ultimately serves as a beacon of hope for the millions affected by Alzheimer’s, driving ongoing efforts towards finding effective treatments.